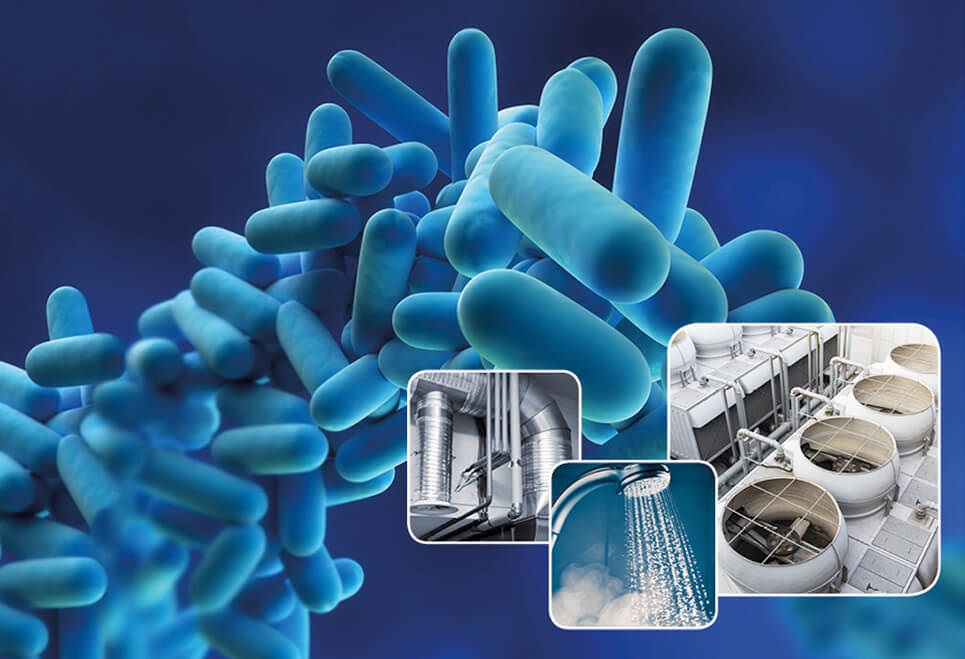
- It eliminates legionella and biofilm
- It does not have the typical smell of chlorine, indeed in the pools it eliminates the smell
- Chlorine dioxide eliminates much more effectively than traditional chlorine, bacteria, viruses, spores, etc.
- The sporicidal and virucidal effect of chlorine dioxide is very high compared to an equal concentration of chlorine
- Chlorine dioxide does not react with ammonium NH4 + or ammonium binders, unlike chlorine which creates amines that have a negative effect to sanitize potable water
- The use of chlorine dioxide prevents the formation of unwanted and harmful substances such as halogen hydrocarbons (Trihanomethani)
- Unlike chlorine, chlorine dioxide has a germ elimination rate that increases with the increase of pH value
Chlorine dioxide
Characteristics of DK-DOX® chlorine dioxide
Demonstration video of two-components chlorine dioxide DK-DOX® activation
Do you want to know more about chlorine dioxide?
Contact us for any information
Contact us